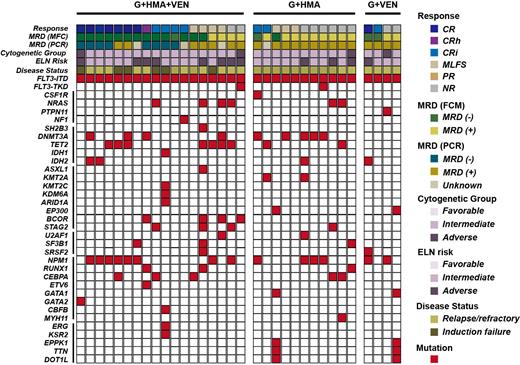
Gilteritinib, a potent FMS-like tyrosine kinase 3 (FLT3) inhibitor, was approved for relapsed/refractory (R/R) FLT3 mutated acute myeloid leukemia (AML) patients but still showed limited efficacy. Here, the efficacy and safety of different gilteritinib-based combination therapy (gilteritinib plus hypomethylating agent [HMA] and venetoclax, G+HMA+VEN; gilteritinib plus HMA, G+HMA; gilteritinib plus venetoclax, G+VEN) in 33 R/R FLT3 mutated AML patients were retrospectively analyzed. Composite complete response (CRc) rate (complete response [CR]+CR with partial hematologic recovery [CRh]+CR with incomplete blood count recovery [CRi]), modified CRc rate (mCRc, CRc+morphologic leukemia-free state [MLFS]), overall survival (OS), duration of remission (DOR), and adverse events were analyzed and compared.The CRc and mCRc rate were 66.7% (12/18) and 88.9% (16/18) in patients who received G+HMA+VEN, which was higher compared with that in G+HMA (CRc: 18.2%, 2/11; mCRc: 45.5%, 5/11) or G+VEN (CRc: 50.0%, 2/4; mCRc: 50.0%, 2/4). The median OS for G+HMA+VEN, G+HMA, G+VEN treatment was not reached, 145.5 days, and 231.0 days, respectively (G+HMA+VEN vs G+HMA, p=0.011). The median DOR for G+HMA+VEN, G+HMA, G+VEN treatment was not reached, 82.0 days, and 77.0 days, respectively (G+HMA+VEN vs G+VEN, p=0.040). Four patients in G+HMA+VEN group received allogenic hematopoietic stem cell transplantation (alloHSCT) after remission exhibited prolonged OS and DOR. The most common grade 3/4 adverse events were cytopenia, febrile neutropenia and pulmonary infection, there were no difference among three groups.In conclusion, our data demonstrated promising response of G+HMA+VEN combination therapy in R/R FLT3 mutated AML patients, and it may consider as an effective bridge to transplantation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal